Real Gas Behavior The Compression Factor (Z) [Example #2]
4.9 (211) In stock


Compressibility factor - Wikipedia

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Compressibility Factor Z
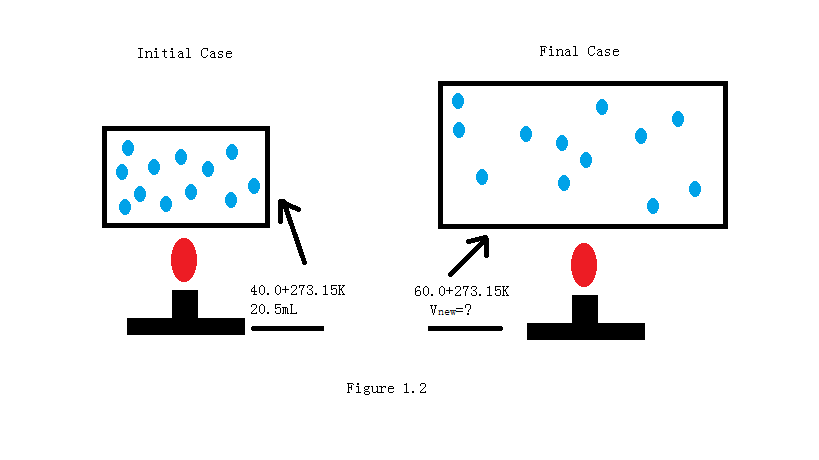
Gas Laws - Overview - Chemistry LibreTexts

Compressibility factor, Z of a gas is given as Z = pV / nRTi What is the value of Z for an ideal gas?ii For real gas what will be the effect

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Physical Chemistry The Compression Factor (Z) [w/1 example]

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

Gas compressibility factor Z: Ideal gas vs Real gas

What is compressibility factor? What is its value for ideal gas
The role of the compressibility factor Z in describing the
Compressibility factor (z): real gases deviate from ideal behav-Turito
Super-critical Fluid Compressibility Factor Z , for Intermediate
 New Womens Striped Adjustable Strap Everyday Plunge Push Up Bras
New Womens Striped Adjustable Strap Everyday Plunge Push Up Bras Fill Station Standard Pillow Protector 2AD (5)
Fill Station Standard Pillow Protector 2AD (5) Imaging Anatomy: Chest, Abdomen, Pelvis: 3rd edition, Siva P. Raman, ISBN: 9780443118005
Imaging Anatomy: Chest, Abdomen, Pelvis: 3rd edition, Siva P. Raman, ISBN: 9780443118005 Cacique Plunge Wire Free Gel Lightly Lined BLUE FLORAL PASTEL Bryant 347699 NWOT
Cacique Plunge Wire Free Gel Lightly Lined BLUE FLORAL PASTEL Bryant 347699 NWOT Woodland Nursery Wall Decor 6 Birch Trees Fox & Friends Fox Deer Owl Squirrel Bunny Raccoon Birds Wall Decal Neutral Nursery Easy to Install
Woodland Nursery Wall Decor 6 Birch Trees Fox & Friends Fox Deer Owl Squirrel Bunny Raccoon Birds Wall Decal Neutral Nursery Easy to Install Samowar, Russisch, Ende 18. / Anfang 19. Jahrhundert Künstler: Russische Meister von Tula Stockfotografie - Alamy
Samowar, Russisch, Ende 18. / Anfang 19. Jahrhundert Künstler: Russische Meister von Tula Stockfotografie - Alamy