Five Common Mistakes Submitting a Premarket Notification
4.8 (327) In stock

How you can avoid the most common errors made when submitting a 510(k), the “premarket notification,” with simple measures

PPT - Premarket Processes & Pathways to Market Pre-amendment, Exempt, 510(k), and 513(g) PowerPoint Presentation - ID:1605402
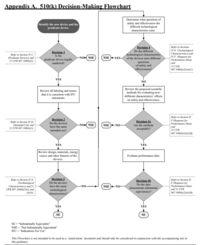
FDA: The 510(k) clearance (Premarket Notification)
FDA 510(k) Submission: A Step-By-Step Guide On How To Prepare Yours

FDA 21 CFR Part 820: 8 Most Common Mistakes to Avoid

Letter-to-File: Dealing With 'Skeletons In The Closet
How To Interpret Nasdaq100 Premarket Indicator - FasterCapital

Avoiding Misbranding: Words Matter When Describing the Regulatory Status of 510(k) Cleared Devices and Registered Device Establishments - Life Sciences Perspectives
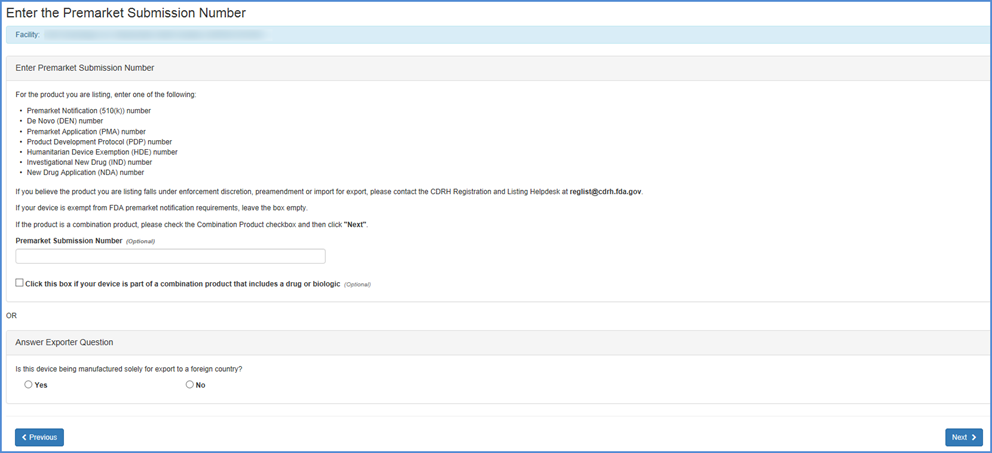
Cancel, Deactivate, or Reactivate a Facility Registration

Premarket Notification The 510(k) Process

Robert A. Allen, PhD on LinkedIn: #biocompatibility #meddevice #medicaldevice #medicaldevices…
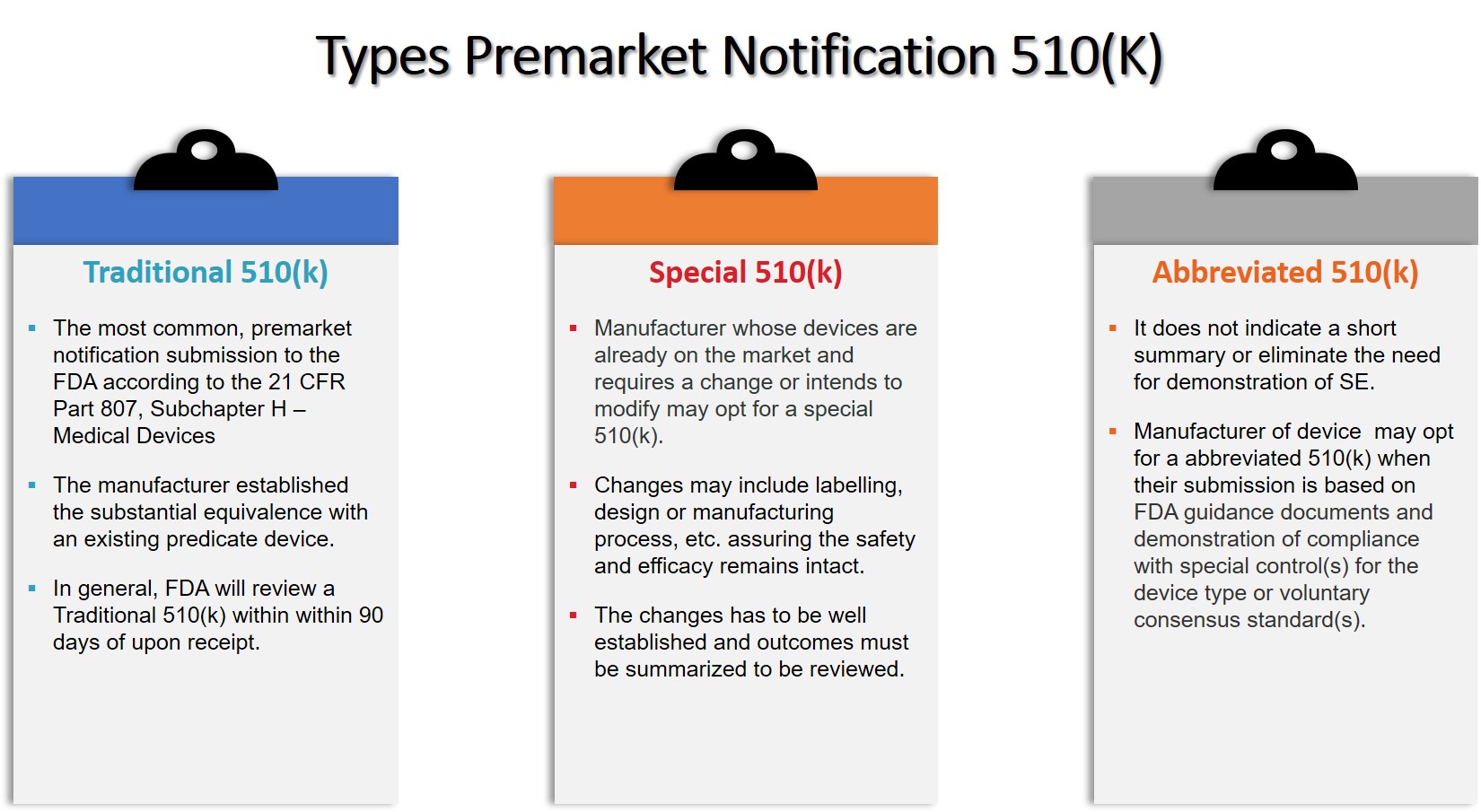
FDA 510k Premarket Notification: Essential Requirements

A Regulatory Perspective: FDA Final Guidance for Design Changes Requiring new 510(k) Submissions
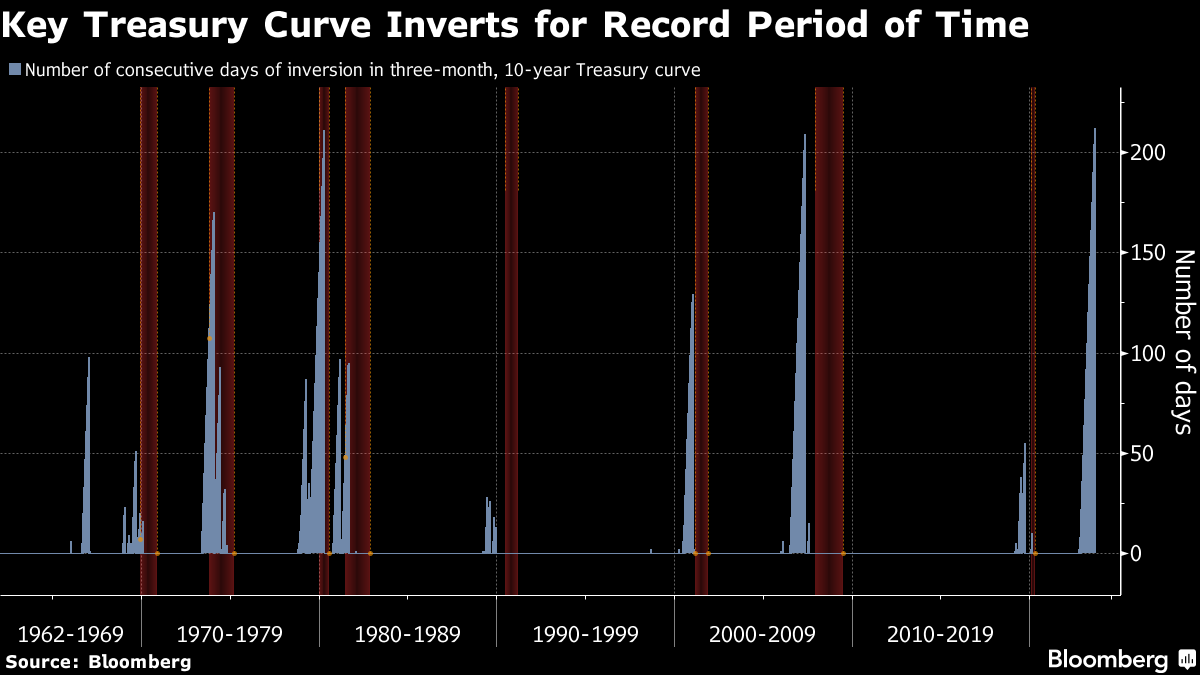
Five Things You Need to Know to Start Your Day - Bloomberg
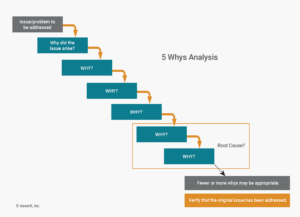
How to Use the 5 Whys for Root Cause Analysis
703 Common Mistakes Royalty-Free Photos and Stock Images
7 Most Common English Grammar Mistakes + TEST - Do you make these
Ten Common Mistakes New Managers Make - Let's Grow Leaders
Most Common Mistakes Vector Icon Badge Stock Vector (Royalty Free
Grammatical Errors: 120 Common Grammar Mistakes in English And How to Avoid Them
 Screen Protector for Line 6 HX Stomp – Ninjafox Engineering
Screen Protector for Line 6 HX Stomp – Ninjafox Engineering Hollow Shaping Tummy Control Boyshort Panties Solid High - Temu
Hollow Shaping Tummy Control Boyshort Panties Solid High - Temu Joe Boxer Women's Push-Up Bras for sale
Joe Boxer Women's Push-Up Bras for sale Womens Shorts Pack Women's Shorts Active Shorts for Women Ladies High Stretch Yoga Sports Womens Boy Short Panties plus Size Short Sleeve Nightshirt Women Short Sleeved Tunic Tops for Women
Womens Shorts Pack Women's Shorts Active Shorts for Women Ladies High Stretch Yoga Sports Womens Boy Short Panties plus Size Short Sleeve Nightshirt Women Short Sleeved Tunic Tops for Women Waist Trainer Xs-10xl Corset Plus Size Waist Trainer Attack Lower
Waist Trainer Xs-10xl Corset Plus Size Waist Trainer Attack Lower Preços baixos em Camisas Bali para Homens
Preços baixos em Camisas Bali para Homens