Thermal Energy & Heat. What is Temperature? Temperature measure
5 (443) In stock

Temperature Conversions o C to o F: o F = 9/5( o C) + 32 o F to o C: o C = 5/9( o F – 32) o C to K: K = o C K to o C: o C = K – 273
What is Temperature Temperature measure of the average KE of all the particles within an object
o F = 9/5( o C) + 32 o F = 9/5(37 o C) + 32 = = 98.6 o F.
Going from Fahrenheit to Celsius Convert 68 o F to o C o C = 5/9( o F – 32) o C = 5/9(68 – 32) = 5/9(36) = 20 o C
Going from Celsius to Kelvin Convert 100 o C to K K = o C K = = 373 K
Going from Kelvin to Celsius Convert 310 K to o C o C = K – 273 o C = 310 – 273 = 37 o C
Thermal Energy the total energy of the particles in a material KE - movement of particles PE - forces within or between particles due to position depends on temperature, mass, and type of substance
B - same temperature, more mass 200 mL 80ºC A 400 mL 80ºC B.
measured in joules (J) a transfer of energy.
80ºC A 10ºC B Heat flows from A to your hand = hot. Heat flows from your hand to B = cold..
Insulators slow the transfer of heat due to air pockets. Conductors easily allow the transfer of heat, like metals. Heat is transferred by conduction, convection, and radiation..
Occurs best in solids. Heat continues to be transferred until both objects reach the same temperature, called a thermal equilibrium..
The cause of wind and weather..
Transferred in all directions. No contact required. Dark or dull objects absorb more than light or shiny objects do..
of 1 kg of material by 1 degree Kelvin units: J/(kg·K) or J/(g·°C).
50 g Al50 g Cu Al - It has a higher specific heat. Al will also take longer to cool down..
Heat Transfer Q = m T C p Q:heat (J) m:mass (g) T:change in temperature (K or °C) C p :specific heat (J/g·K or J/g.o C) T = T f - T i – Q = heat loss + Q = heat gain
Heat Transfer Calorimeter device used to measure changes in thermal energy Coffee cup Calorimeter in an insulated system, heat gained = heat lost
How much heat is lost by the spoon. GIVEN: m = 32 g T i = 60°C T f = 20°C Q = . C p = 235 J/kg·K WORK: Q = m· T·C p m = 32 g = kg T = 20°C - 60°C = – 40°C T = 293 K – 333 K = -40 K Q = (0.032kg)(-40 K)(235J/kg·K) Q = J (lost heat, negative).
GIVEN: m = 230 g T i = 12°C T f = 90°C Q = . C p = J/g· o C WORK: Q = m· T·C p m = 230 g T = 90°C - 12°C = 78°C Q = (230 g)(78 o C)(4.184 J/g· o C) Q = 75,061 J (gained heat, positive).
Why is temperature measured in Kelvin and heat in joule? - Quora

Specific heat, Definition & Facts

Lab Get ready for the …. Examples of Heat Transfer by Conduction Define Conduction. - ppt download

Thermal Energy, Temperature and Heat - Student Lesson Outline

Lab Get ready for the …. Examples of Heat Transfer by Conduction Define Conduction. - ppt download
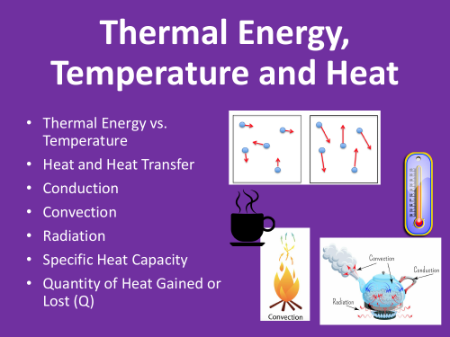
Thermal Energy, Temperature and Heat

Thermal Energy & Heat. What is Temperature? Temperature measure of the average KE of all the particles within an object. - ppt download
Lab Get ready for the …. Examples of Heat Transfer by Conduction Define Conduction. - ppt download

11.2.2 Temperature, Heat, and Internal Energy – xmPhysics
360Science™: Thermal Energy and Heat Transfer
Convert 36.3 Celsius To Fahrenheit
Temperature Measurement Units, Overview & Conversion - Lesson
 Watch Selena Gomez Strike A Pose In PUMA's Amp XT Sneaker
Watch Selena Gomez Strike A Pose In PUMA's Amp XT Sneaker Tweed blazer Talbots Purple size 4 US in Tweed - 26694670
Tweed blazer Talbots Purple size 4 US in Tweed - 26694670 Nike Running. Nike CA
Nike Running. Nike CA TOP 10 BEST Bra Fitting in Aurora, ON - March 2024 - Yelp
TOP 10 BEST Bra Fitting in Aurora, ON - March 2024 - Yelp Supreme 2019-20FW Unisex Street Style Skater Style Boxer
Supreme 2019-20FW Unisex Street Style Skater Style Boxer 3 Sets Large Silicone Plant Molds, Container Coaster Resin Concrete Mold, Cylinder Cube Hexagon Silicone Mold for Candle Vessel Succulent Planter Pot
3 Sets Large Silicone Plant Molds, Container Coaster Resin Concrete Mold, Cylinder Cube Hexagon Silicone Mold for Candle Vessel Succulent Planter Pot