Compressibility factor (Z) for a van der Waals real gas at
4.9 (282) In stock

Share your videos with friends, family and the world

Derivation of Van Der Waals Equation

Compressibility factor - Wikipedia

Objectives_template

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced
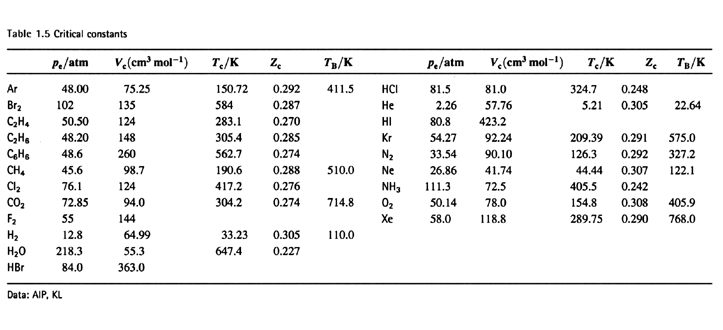
Chapter 1 Properties of Gases

Solved We showed, for a van der Waals gas, that the

JEE: Van der Waals Equation, Chemistry By Unacademy
Under what conditions do you expect a real gas such as hydrogen gas to behave like an ideal gas? - Quora

Real Gases and the van der Waals Equation Explained

Solved 9 Compression factor Z Use the van-der-Waals equation
What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
Compressibility factor (gases) - Knowino
Explain how the compression factor varies with pressure and
What is the compressibility factor (Z) for 0.02 mole of a van der
- George Womens White Padded T-Shirt Bra 32F
 Under Armour - Mujer Coolswitch Rn Para Mujer Zapatillas De Training - Ss17 Gimnasio Morado < Young Ukuleles
Under Armour - Mujer Coolswitch Rn Para Mujer Zapatillas De Training - Ss17 Gimnasio Morado < Young Ukuleles Everyday Bras Wirefree Silk Bralettes for Women Comfort Fit Camisole Bra French Triangle Bras Summer Thin Underwear (Color : 2pcs d, Size : Large) : : Clothing, Shoes & Accessories
Everyday Bras Wirefree Silk Bralettes for Women Comfort Fit Camisole Bra French Triangle Bras Summer Thin Underwear (Color : 2pcs d, Size : Large) : : Clothing, Shoes & Accessories Leggings rossi lucidi push up con zip frontale tessuto tecnico Canoan.
Leggings rossi lucidi push up con zip frontale tessuto tecnico Canoan. 7 tips para hacer ejercicio todos los días
7 tips para hacer ejercicio todos los días Womens Liliana Faux Leather Leggings in Olive Green Size Small by Fashion Nova
Womens Liliana Faux Leather Leggings in Olive Green Size Small by Fashion Nova
