OneClass: For a real gas, the compressibility factor, Z, is
4.8 (694) In stock


Energies, Free Full-Text

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Real Gases and Compressibility Factor

OneClass: At low pressures the compressibility factor for a Van der Waal's gas is given by Z-1+[b- (a

OneClass: For a real gas, the compressibility factor, Z, is defined as Z (T, P) = PV/nRT For an ideal
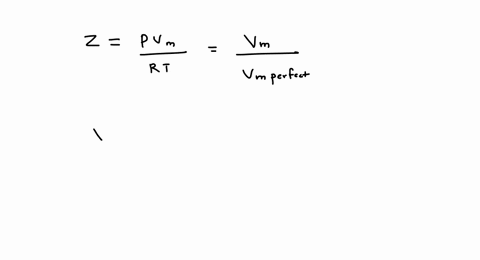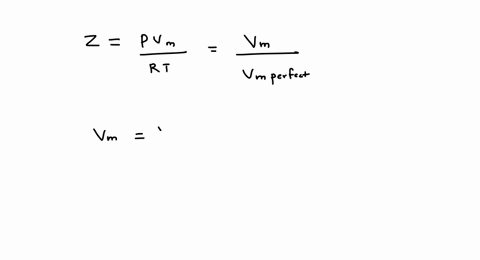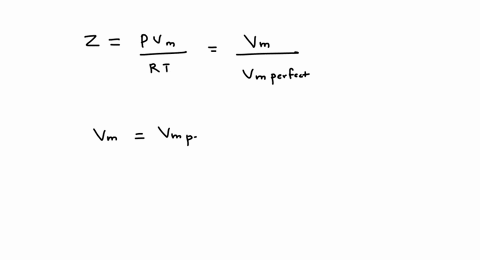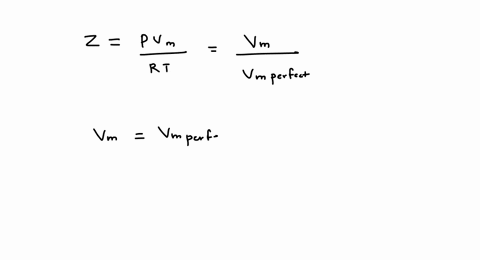
SOLVED: A gas at 350 K 15 atm has a molar volume 12 percent smaller than that calculated from the perfect gas law. Compressibility factor under these conditions can be expressed in

Description of real gases: Compression factor

Mechanics and physics of the light-driven response of hydrogels - ScienceDirect

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

Determine Compressibility of Gases

Physical Chemistry The Compression Factor (Z) [w/1 example]

OneClass: At low pressures the compressibility factor for a Van der Waal's gas is given by Z-1+[b- (a

For a gas at a given temperature, the compression factor is described by the empirical equa - OneClass
Math cad compressibility factor, z, of real gas using the redlich
Compressibility Factor Z for sub-critical pressures for Lee
Compressibility factor (z): real gases deviate from ideal behav-Turito
Compressibility factor - Wikiwand
Compressibility Factor, z vs Pressure, P (kPa), line chart made by Jdvani
 HGps8w Women's Bandeau Bra Plus Size Strapless Stretchy Soft Brarette Seamless Removable Pads Bandeau Tube Top Bras, 3 Pack at Women's Clothing store
HGps8w Women's Bandeau Bra Plus Size Strapless Stretchy Soft Brarette Seamless Removable Pads Bandeau Tube Top Bras, 3 Pack at Women's Clothing store lululemon athletica, Pants & Jumpsuits, Lululemon Wunder Under Highrise 78 Tight 25
lululemon athletica, Pants & Jumpsuits, Lululemon Wunder Under Highrise 78 Tight 25 Top 10 Women's Rehab Centers In The U.S. - Addiction Resource
Top 10 Women's Rehab Centers In The U.S. - Addiction Resource Sentirse libre y atrevida, cuando salgas a la calle, cuando
Sentirse libre y atrevida, cuando salgas a la calle, cuando Plus size women's merino wool leggings - Black - Dilling
Plus size women's merino wool leggings - Black - Dilling Zarely, Accessories
Zarely, Accessories