Consider the graph between compressibility factor Z and pressure P
4.6 (65) In stock

Z1 means force of attraction dominating ie a is considerable b can be negligible at low temperature and low pressure Lower is the value of Z easier is the process of liquification
The compressibility factor is actually a factor that corrects the actual value of the gas versus the ideal gas. Let us learn and understand this concept.
Watch this video to understand the behaviour of real gases with the help of the compressibility factor. This is an important topic for JEE main.
What is the compressibility factor, and how does it vary with an increase in temperature and pressure? Watch this video to get the answer. This is an importa

If the uncertainty in the position of a particle is equal to its de-Br

Chemistry Desk: Effect of Pressure

PDF) ACT- All Goa Chemistry Quiz - Std.XI - December 2017actgoa.weebly.com/uploads/3/7/2/3/37238293/act_xi_20171 ACT- All Goa Chemistry Quiz - Std.XI - December – 2017 Date: 18/12/17
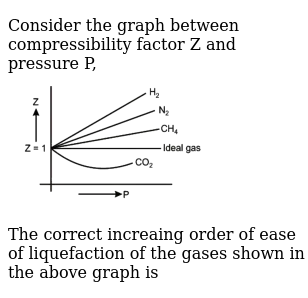
Consider the graph between compressibility factor Z and pressure P

Compressibility factor of water vapor along its saturation curve. Error

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

pH of a 100 cc solution is 2. It will not change if
What is the significance of the curve part in Z vs. P graph of compressibility of a gas? - Quora

Praveen-Fl (22-23) MCT - 1, PDF, Acceleration
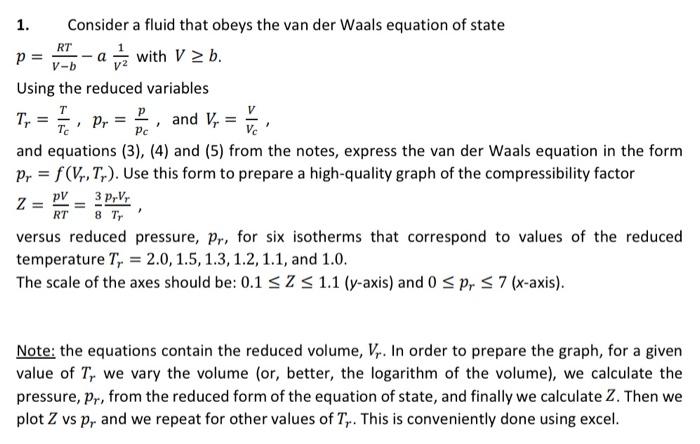
1. Consider a fluid that obeys the van der Waals
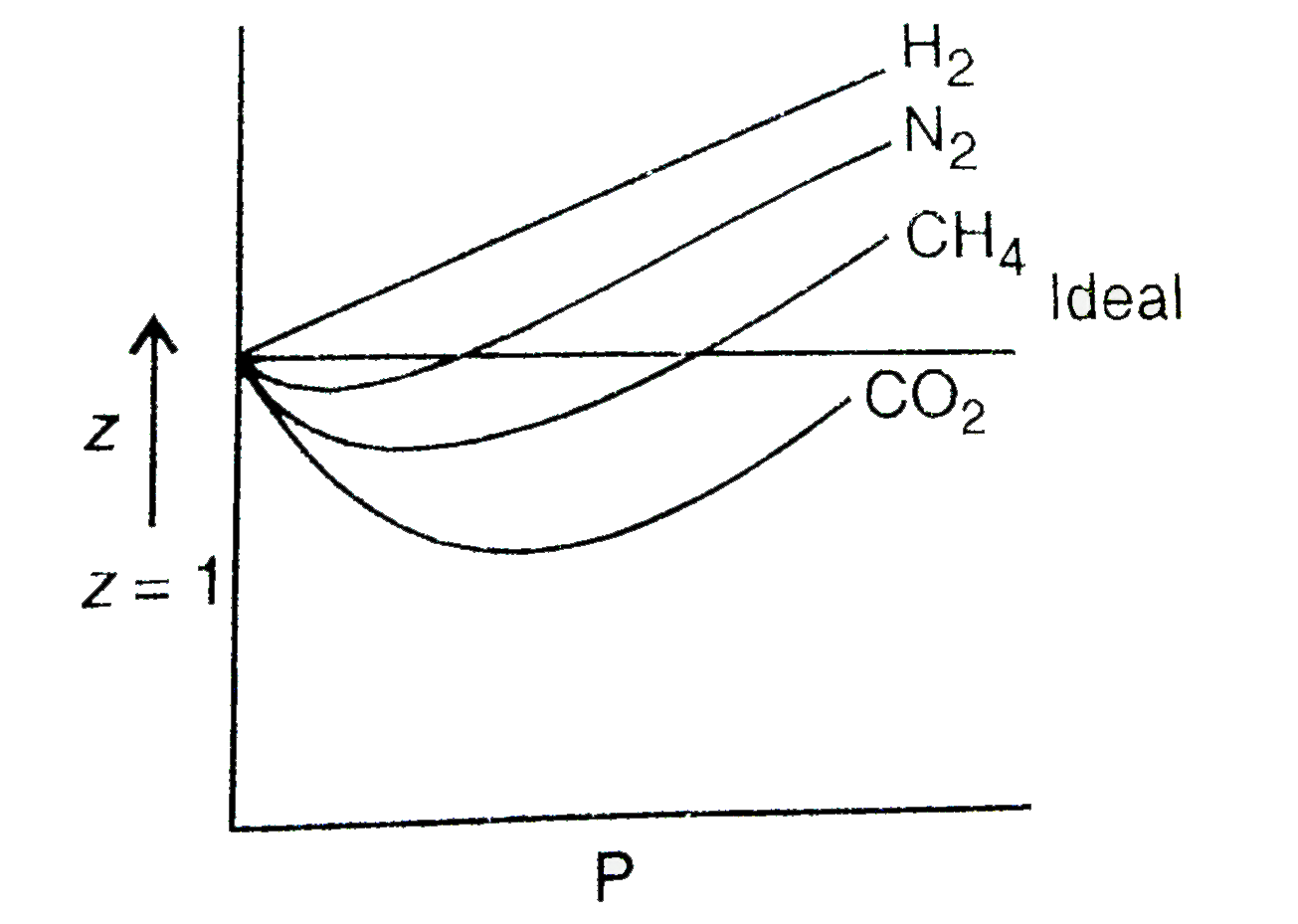
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
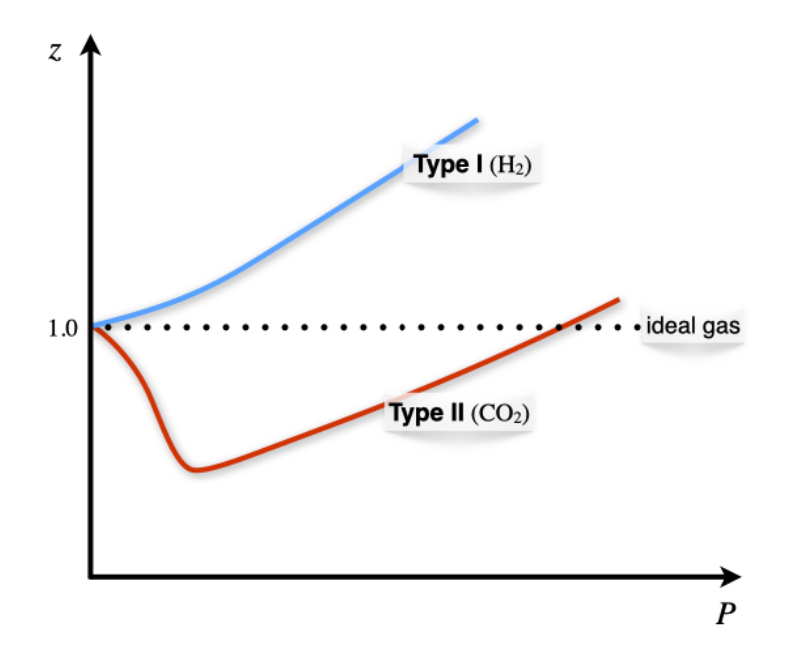
11.3: Critical Phenomena - Chemistry LibreTexts

Compressibility factor - Wikipedia
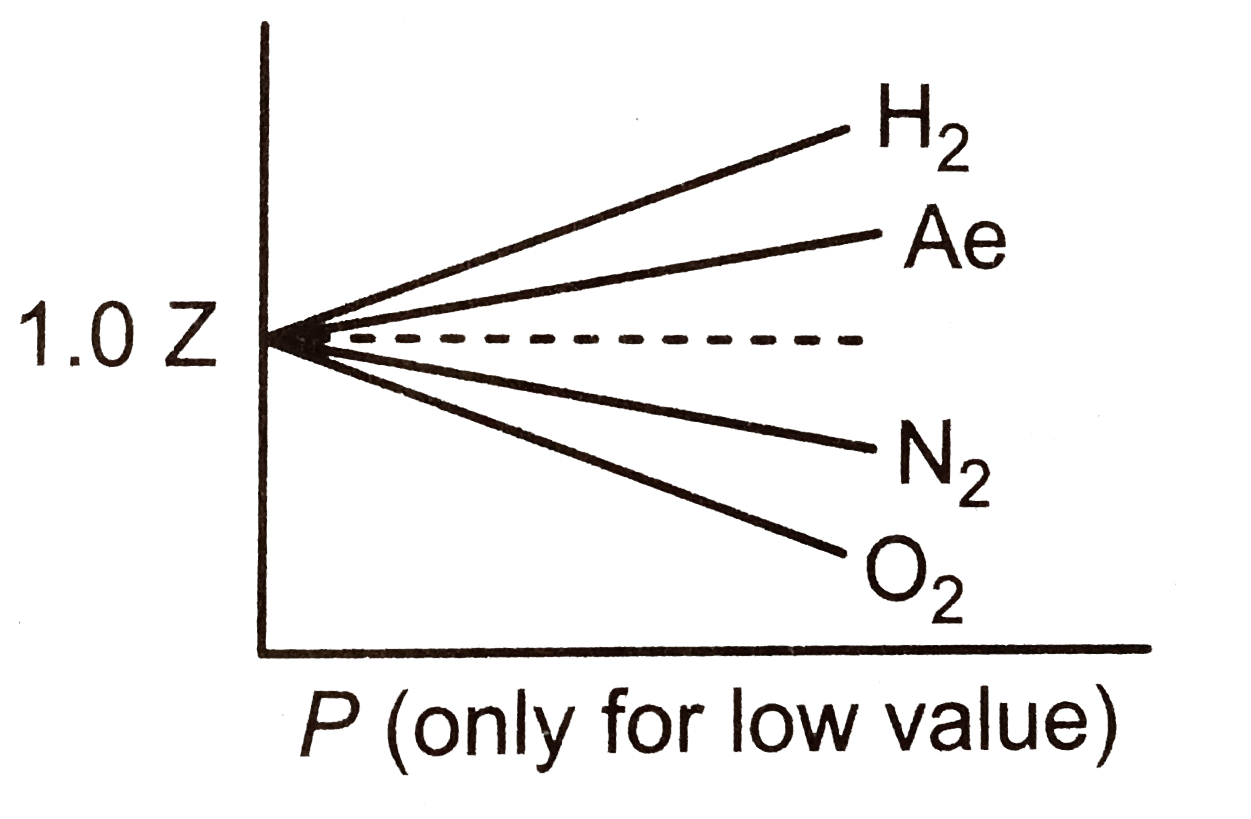
The intercept on y-axis leads to a value of 'n' .

gas laws - How to find the temperature relationship between the isotherms in a compressibility factor (Z) vs pressure graph? - Chemistry Stack Exchange
Compressibility Factor Calculator - File Exchange - MATLAB Central
Compressibility Factor Charts - Wolfram Demonstrations Project
Gas Compressibility - an overview
The compressibility factor Z a low-pressure range of all gases





