Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart from ideal. - ppt download
4.6 (73) In stock
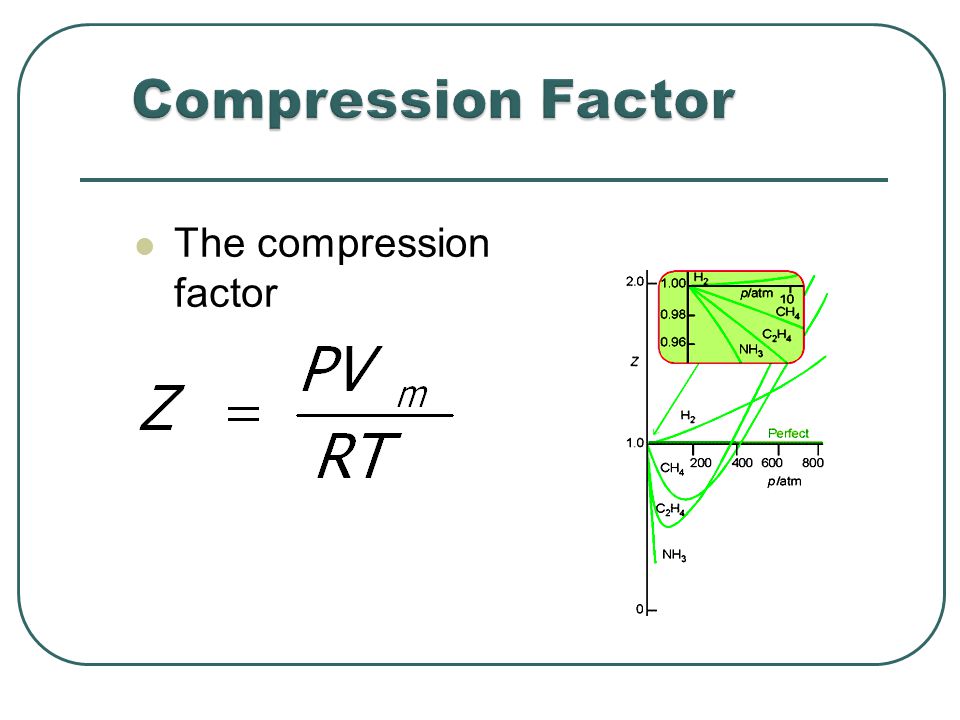
Most real gases depart from ideal behaviour at deviation from low temperature high pressure.
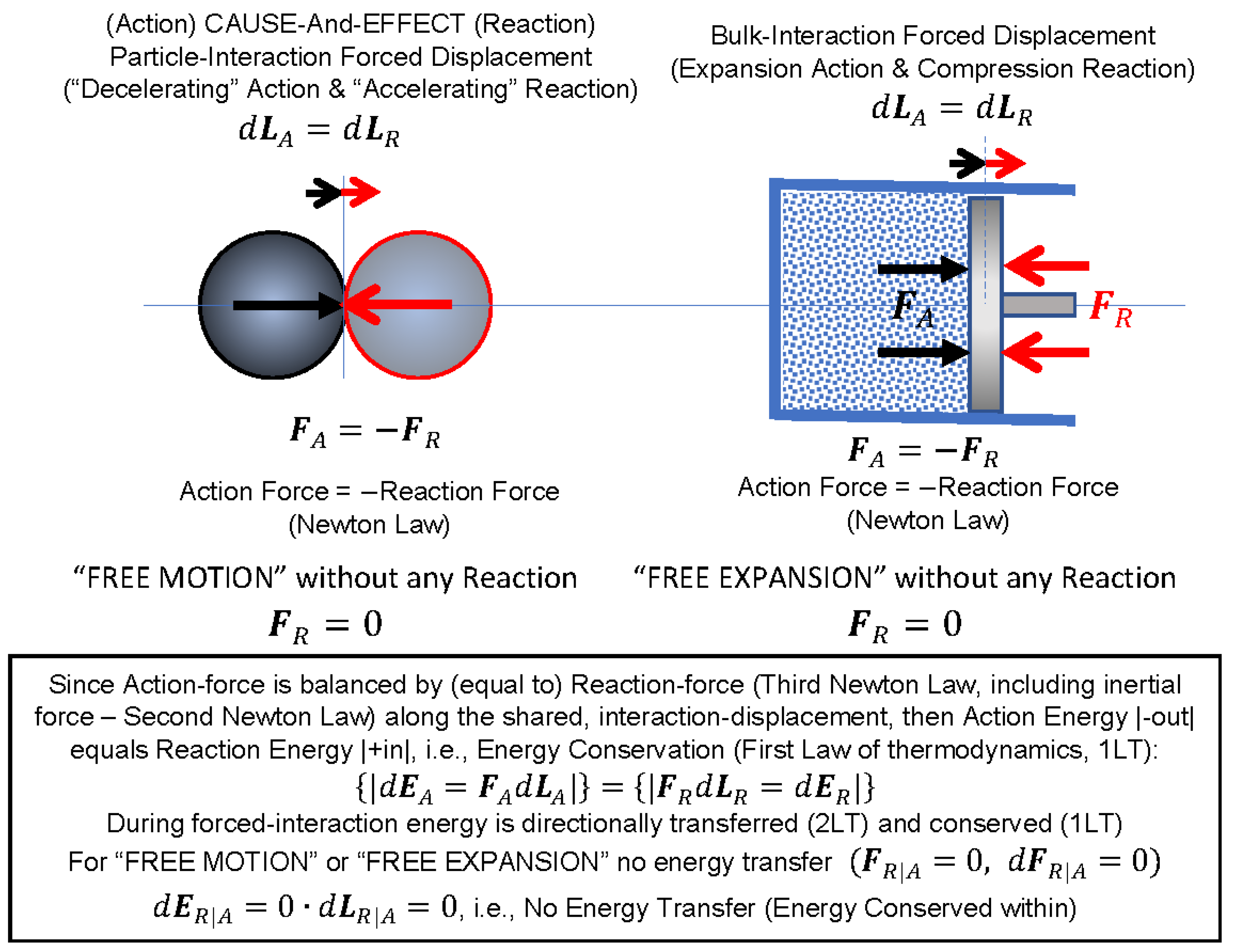
High positive potential energy (little separation) Repulsive interactions Intermediate separations attractive interactions dominate Large separations (on the right) the potential energy is zero and there is no interaction between the molecules..
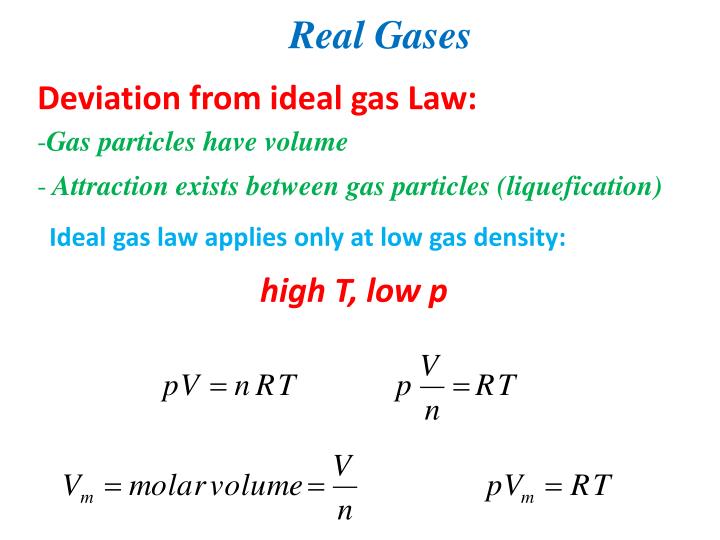
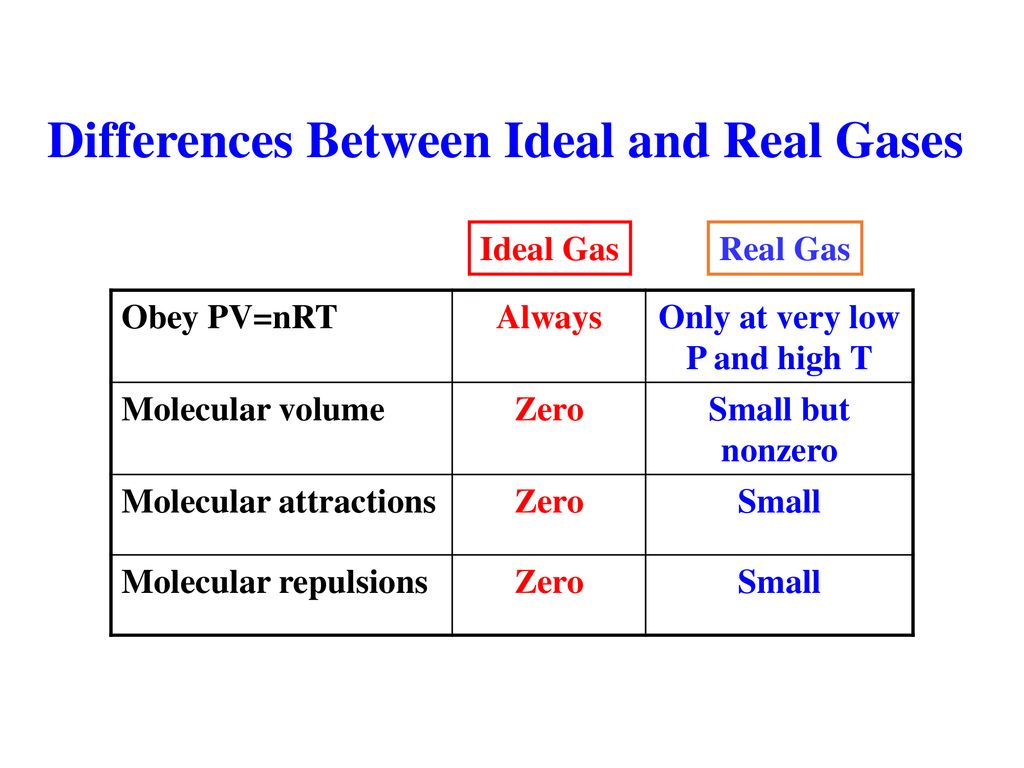
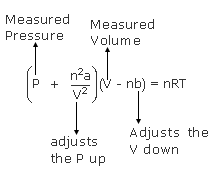
Real gas molecules do attract one another (P id = P obs + constant) Real gas molecules are not point masses (V id = V obs - const.)
V id = V obs - nb b is a constant for different gases P id = P obs + a (n / V) 2 a is also different for different gases Ideal gas Law P id V id = nRT
Critical temperature (T c ) - the temperature above which a gas cannot be liquefied Critical pressure (P c ) – the minimum pressure that needs to be applied at T c to bring about liquefaction
For a perfect gas, the slope is zero Boyle temperature the slope is zero and the gas behaves perfectly over a wider range of conditions than at other temperatures.
Boyle temperature - for a van der Waal s gas, the Boyle temperature (T B ) is written
The reduced state variables are defined
Re-write the Van der Waals in terms of reduced variables
The chemical potential of a real gas is written in terms of its fugacity
In gaseous systems, we relate the fugacity (or activity) to the ideal pressure of the gas via.
Define the fugacity coefficient = f / P For a real gas.
Comparing the chemical potential of the real gas to the chemical potential of an ideal gas at the same pressure
The fugacity coefficients are obtained from the compression factors (Z) as shown below
PPT - Real Gases PowerPoint Presentation, free download - ID:3450550
Real Gases Real molecules do take up space and do interact with each other (especially polar molecules). Need to add correction factors to the ideal gas. - ppt download
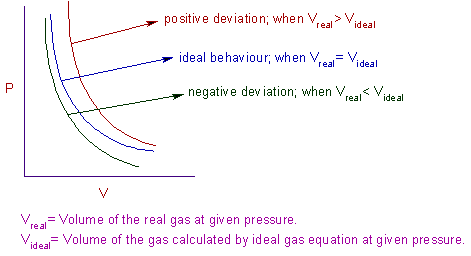
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
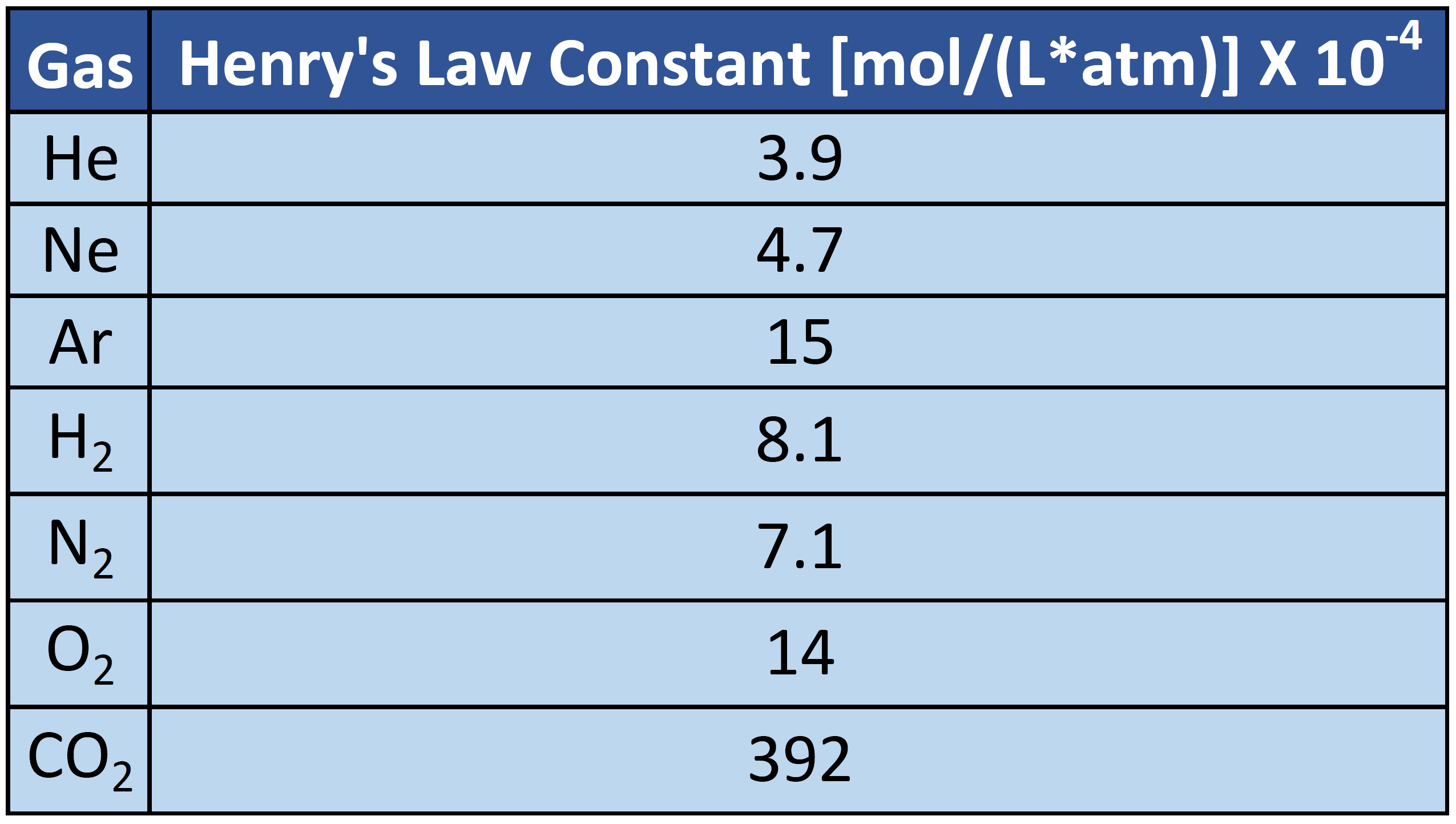
CH104: Chapter 7 - Solutions - Chemistry

Chemistry 231 Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart. - ppt download

PPT - KMT, Graham's Law & Real Gases PowerPoint Presentation - ID:942282
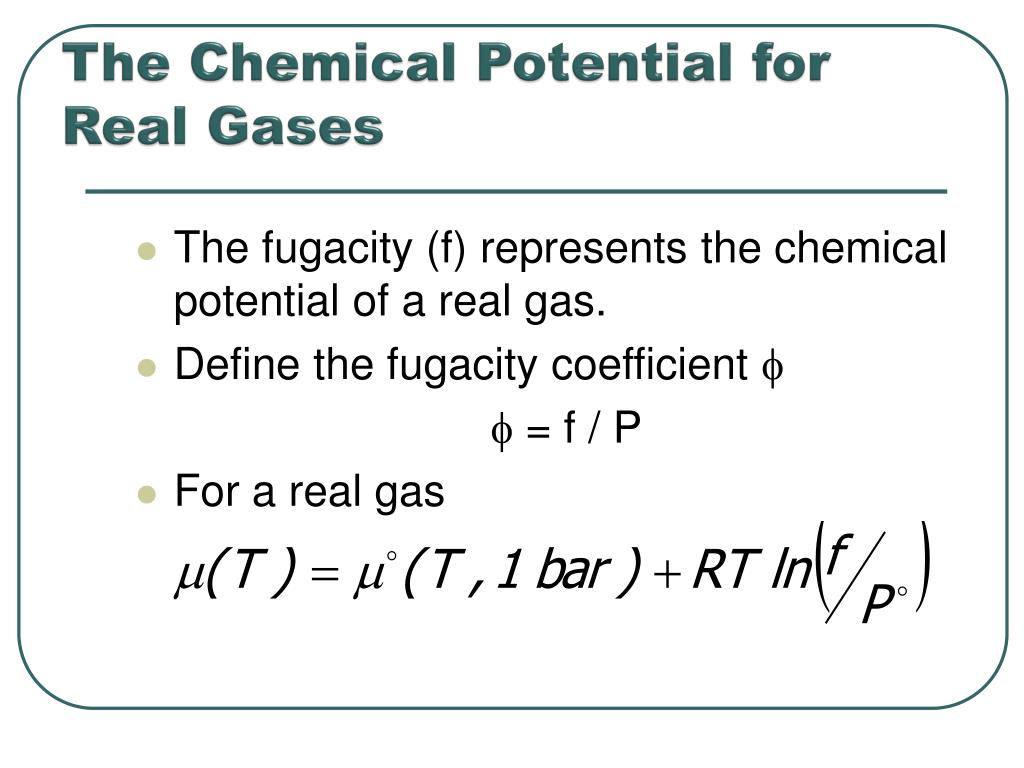
PPT - Chemistry 231 PowerPoint Presentation, free download - ID:967425
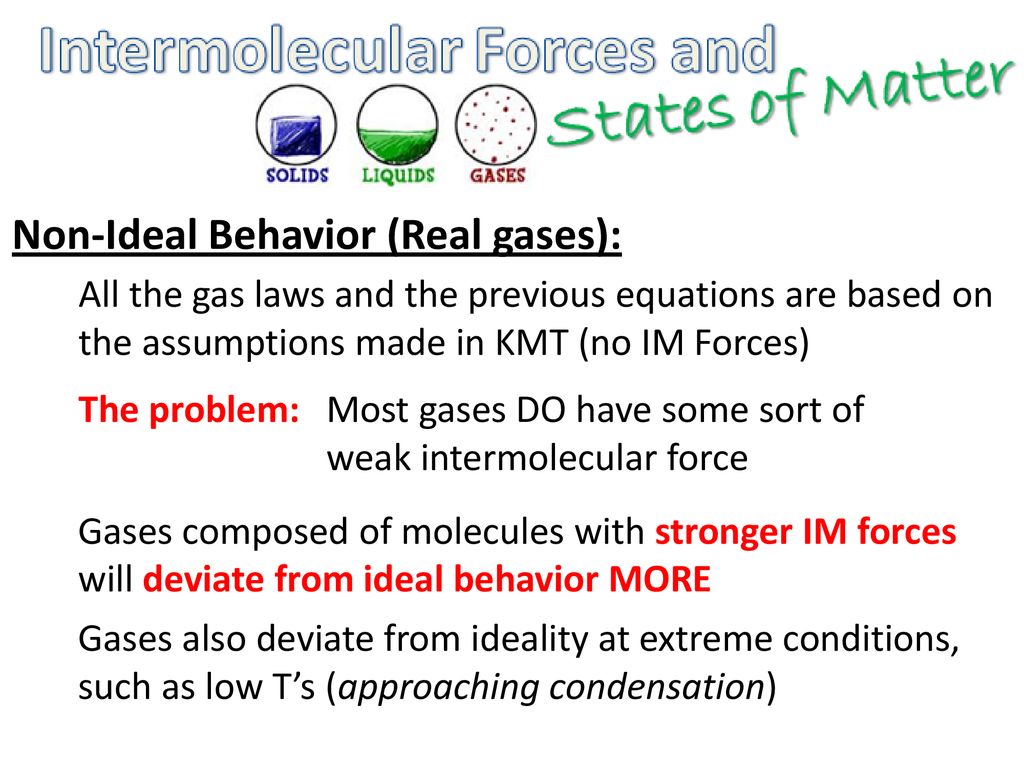
Non-Ideal Behavior (Real gases): - ppt download

Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart from ideal. - ppt download

Ideal and Real Gas
Entropy, Free Full-Text

Chemistry 231 Real Gases. The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. Most real gases depart. - ppt download
Vanderwall's Equation
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
Answer in General Chemistry for Carl #275533
SOLVED: For a gas at a given temperature, the compression factor