SOLVED: Derive an expression for the compression factor of a gas
4.9 (596) In stock

VIDEO ANSWER: And this question we're going to be dealing with the equation state equation of state where P multiplied by V minus n B. Is equality and are a team. So we're dealing with a scenario where VM is equal to 10 B. So what would have right
Derive an expression for the compression factor of a gas that obeys the equation of state p(V - nb) = nRT, where b and R are constants. If the pressure and temperature are such that Vm = 10b, what is the numerical value of the compression factor?
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
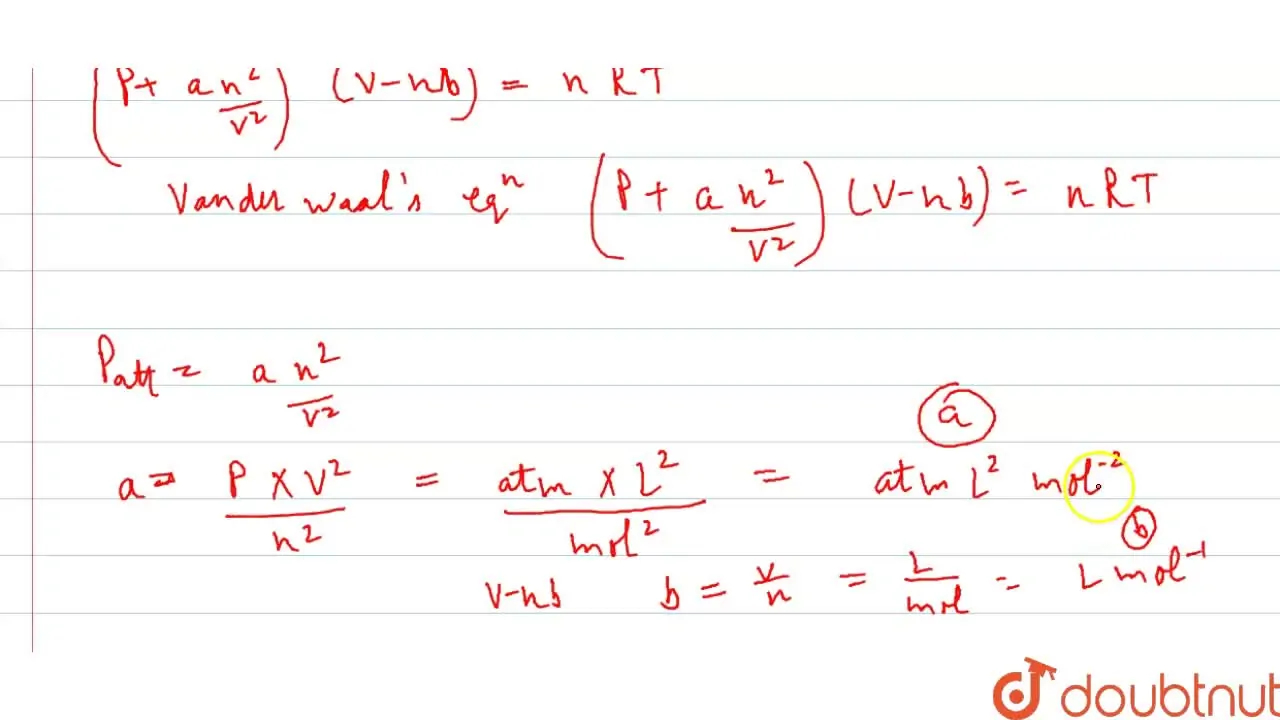
Punjabi] Derive van der Waals' equation of State for n moles of gas.

COMPRESSIBILITY FACTOR
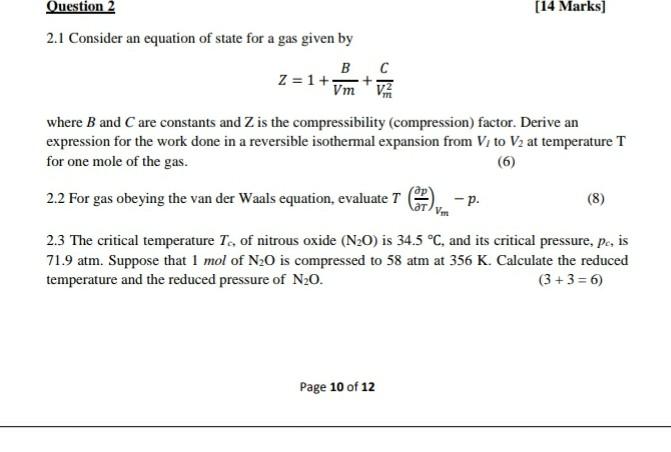
Solved Question 2 (14 Marks] 2.1 Consider an equation of

Compression Factor - an overview
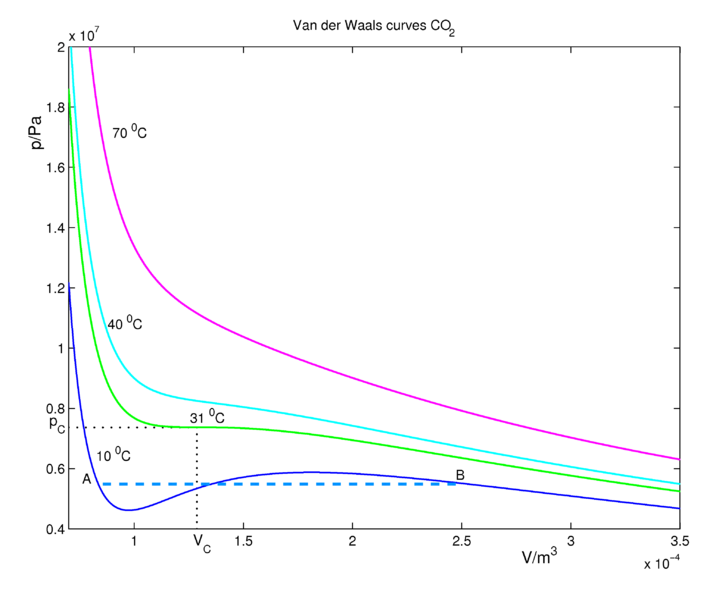
16.3: A Cubic Equation of State - Chemistry LibreTexts
Isentropic exponent κ for hypothetical natural gas mixture with

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
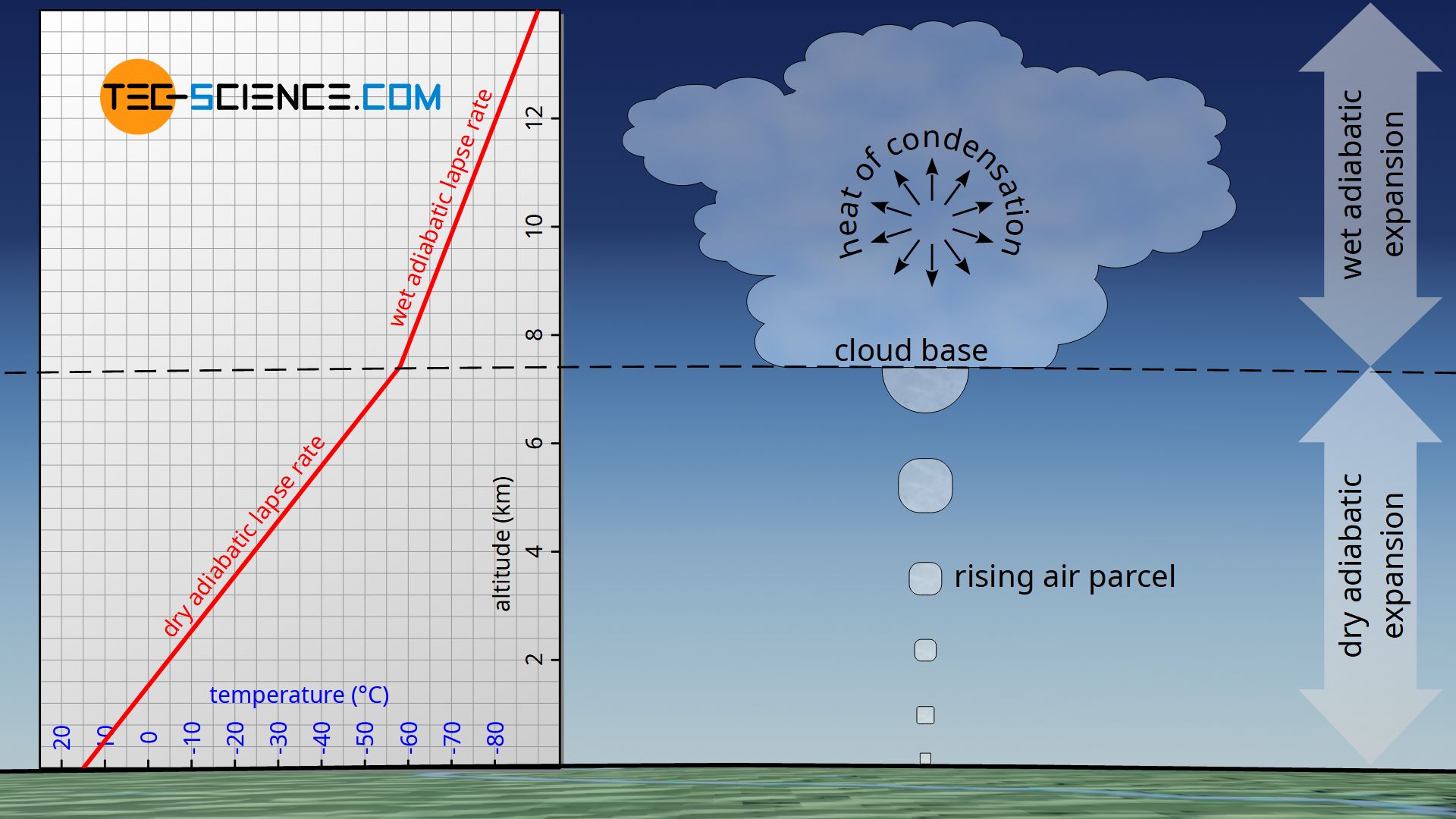
Derivation of the barometric formula (adiabatic atmosphere) - tec
How to Calculate Compression Ratio: 9 Steps (with Pictures)
Answer in General Chemistry for Carl #275533
- Serta Singapore - SERTA GSS SPECIAL - Purchase Perfect Sleeper - Endless Comfort and Enjoy FREE Serta Bedding Accessories worth SGD 499 + FREE Storage Bed worth SGD 1,588 *while stocks last* #Serta #PerfectSleeper #AlwaysComfortable
 Sports Bra Sewing Pattern All Sizes. One Price. Digital Download
Sports Bra Sewing Pattern All Sizes. One Price. Digital Download Leggings Under Armour UA Rush Legging
Leggings Under Armour UA Rush Legging WMNS) Gucci Monogram Tights 'Grey' 691619-3GAHR-1281 - KICKS CREW
WMNS) Gucci Monogram Tights 'Grey' 691619-3GAHR-1281 - KICKS CREW Dos jóvenes mejoran la vida de los ostomizados con fundas pioneras para las bolsas - Noticias de enfermería y salud
Dos jóvenes mejoran la vida de los ostomizados con fundas pioneras para las bolsas - Noticias de enfermería y salud Underwear Briefs Bag Hanger Bra Storage Hanging Bag Wall Fabric Shoes Wardrobe Organizer Wall Shelf Organizer Socks Closet Door - AliExpress
Underwear Briefs Bag Hanger Bra Storage Hanging Bag Wall Fabric Shoes Wardrobe Organizer Wall Shelf Organizer Socks Closet Door - AliExpress
